The US Food and Drug Administration (FDA) recently revealed a proposal to introduce a front-of-pack nutrition label for most packaged foods to aid consumers in identifying healthier food options. This initiative is part of the US government’s efforts to address chronic diseases like heart disease, diabetes, and cancer, which are major causes of death and disability in the country.
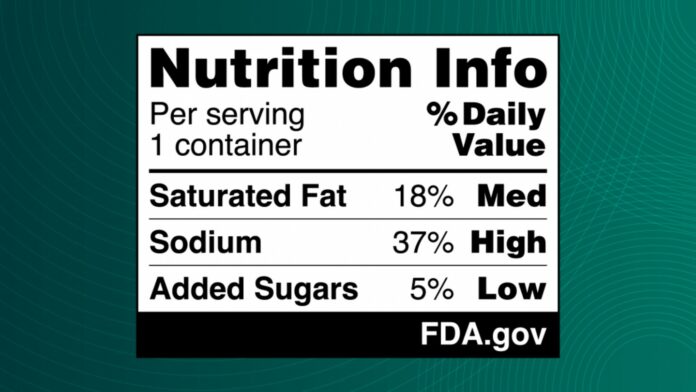
In 2022, US President Joe Biden announced a plan to combat diet-related diseases by implementing a front-of-package labeling system. The proposed Nutrition Info box will indicate whether a product’s saturated fat, sodium, and added sugars are categorized as “low,” “medium,” or “high,” similar to the existing Nutrition Facts label.
Under the FDA’s proposal, manufacturers with annual sales exceeding $10 million will have three years to comply, while smaller businesses will have four years. The FDA has also opened a feedback period until May 16th to gather input from stakeholders.
FDA commissioner Robert Califf emphasized the importance of making nutrition information easily accessible to consumers, stating that the new labeling system would simplify the process of choosing healthier foods. The FDA cited studies indicating that many ultra-processed foods in the US contain high levels of saturated fat, sodium, and added sugars.
Industry response to the proposal has been mixed. Jennifer Hatcher, chief public policy officer of the Food Industry Association (FMI), praised the scheme but raised concerns about the practical challenges of implementation. She highlighted the need for redesigning packaging labels to accommodate the new requirements, potentially displacing crucial information such as date labels.
Dr. Peter Lurie, president of the Center for Science in the Public Interest, hailed the proposal as long overdue, emphasizing its potential to drive healthier consumer choices and encourage companies to produce more nutritious products. He also urged the FDA to consider international evidence supporting similar labeling systems adopted in other regions.
While the proposal has drawn support from public health advocates, concerns remain about its impact on packaging redesign costs and the overall effectiveness in promoting healthier food choices. As the FDA continues to refine the policy, stakeholders across the food industry will closely monitor developments to assess the potential implications on consumer behavior and public health outcomes.
In conclusion, the introduction of front-of-pack nutrition labeling represents a significant step towards empowering consumers to make informed dietary choices and combatting the prevalence of diet-related chronic diseases in the US. By providing clear and accessible information on key nutritional components, the FDA aims to drive positive changes in food consumption patterns and contribute to improved public health outcomes.